
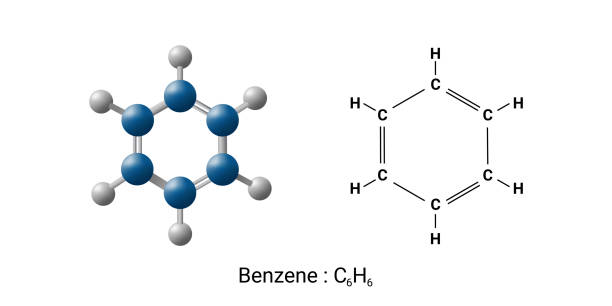
Benzene is an essential organic chemical compound with the molecular formula C₆H₆. It consists of six carbon atoms arranged in a planar ring, with one hydrogen atom attached to each carbon. As it contains only carbon and hydrogen atoms, benzene is classified as a hydrocarbon—specifically, an aromatic hydrocarbon due to its stable ring structure.
The name benzene is derived from gum benzoin, an aromatic resin well known to European pharmacists and perfumers since the 16th century. By the 1800s, sublimation of benzoin produced an acidic compound known as flowers of benzoin, now recognized as benzoic acid.
In 1836, French chemist Auguste Laurent coined the name “phène” for benzene—a root word still seen today in terms such as phenol (hydroxylated benzene) and phenyl, the radical formed when one hydrogen atom is removed from benzene.
Appearance: Colorless, volatile liquid
Odor: Sweet aromatic smell (responsible for the smell at gas stations)
Flammability: Highly flammable
Solubility: Slightly soluble in water, miscible with organic solvents
Health Impact: Classified as a Group 1 human carcinogen by the IARC
Benzene occurs naturally in crude oil and is also produced during the combustion of organic material. Today, it is primarily manufactured as a byproduct of oil refining and steam cracking in petrochemical plants.
Benzene serves primarily as a feedstock for the manufacture of more complex chemicals:
Ethylbenzene – Precursor to styrene, used to produce polystyrene plastics and EPS foam.
Cumene – Used to make phenol and acetone, important for resins, adhesives, and plastics.
Cyclohexane – Intermediate for nylon fiber production.
Nitrobenzene – Used in aniline production for dyes and rubber chemicals.
Alkylbenzene – Base for detergents.
In total, over 50% of global benzene output is used to produce ethylbenzene, followed by cumene (~20%) and cyclohexane (~10%).
Other applications include:
Lubricants
Rubber manufacturing
Pesticides
Pharmaceuticals
Explosives
Dyes and colorants
As of recent years:
China is the leading consumer of benzene.
The Middle East and Africa are rapidly expanding production capacities.
Western Europe and North America show relatively stagnant production due to stricter environmental regulations.
Toluene is often used as a less toxic substitute for benzene in various applications:
Similar solvent properties
Used as a fuel additive (enhances octane rating)
Precursor for producing benzene itself
Toluene has a wider liquid range and lower toxicity, making it more suitable for non-industrial applications.
Benzene is toxic to humans and can lead to:
Short-term exposure: Drowsiness, dizziness, unconsciousness
Long-term exposure: Bone marrow suppression, leukemia, immune system damage
Due to these risks, non-industrial and consumer uses of benzene have been largely phased out.
While standards may vary by region and application, industrial benzene must meet strict purity and safety requirements. Common parameters include:
Purity: ≥ 99.85%
Boiling Point: 80.1°C
Melting Point: 5.5°C
Density: 0.8765 g/cm³ at 15°C
Refractive Index: 1.5011
Sulfur Content: ≤ 1 ppm
Regulatory bodies like ASTM, ISO, and EU REACH define safe handling, transport, and exposure limits.
Benzene remains an indispensable chemical building block in the petrochemical industry. Its unique properties make it vital for producing a wide array of materials, including plastics, resins, synthetic fibers, and specialty chemicals. However, due to its carcinogenic nature, strict controls govern its usage and exposure.
As the global demand for downstream derivatives grows—especially in Asia and the Middle East—benzene’s role as a petrochemical feedstock will remain central to modern industrial chemistry.